Supply Chain of Pharmaceuticals: Sensitive, expensive, high-tech
| Written by Constance Stickler
Medicine, and with it drugs, goes far back into human history. But it was only with the uniting of single apothecaries, their collaboration with chemical laboratories, significant discoveries and, last but not least, the possibilities of mass production and refrigerated transport that we were able to create the healthcare environment as we know it today.
In this article, we discuss the complexity of the supply chain of pharmaceuticals.

No video selected
Select a video type in the sidebar.
A brief historical outline
We find the oldest collection of recipes with the Sumerians in Mesopotamia, around 3,000 BC, which is 5,000 years old today. One thousand five hundred years later, there are already 880 remedies in the Egyptian Ebers papyrus. The extensive medical knowledge of the ancient Egyptians is also reflected in numerous well-preserved mummies.
The first pharmacies emerged in hospitals in Baghdad (Iraq) in the 9th century. In the centuries that followed, monks from southern Italy and Spain brought knowledge to Europe, which had a great deal of catching up after the Roman Empire's collapse.
The first form of drug delivery was travelling dealers who went from door to door with their products packed in clay jars, glass bottles and chipboard boxes.
In the 19th century, small apothecary shops started working with dye and chemical companies and founded chemical labs. With joint forces, they were able to develop more effective products for a greater range of applications. For a long time, patients could buy medicines without a prescription. Products like "Cocaine Toothache Drops" were not patented, and their formulas were usually a secret. After some tragic events, authorities requested preclinical and clinical tests, and drugs had to be approved by administrative institutions.
The pharmaceutical industry grew and celebrated successes like insulin's invention and mass production in the 1930s. Finally, diabetes was no longer a fatal diagnosis. Twenty years later, quite a range of antibiotics was available, as well as a vaccine to fight polio, one of the most worrying childhood diseases at that time.
Today a vast number of medicines is offered, and the total revenues for pharmaceutical products were 1.42 trillion USD in 2021. (source.)
However: About seven in ten products need temperature-controlled storage and shipping.
Why is precise temperature so critical along the cold chain of pharmaceuticals?
The temperature has a direct impact on the rate of oxidation and hydrolysis. As a rule of thumb, the rate of reactions increases with increasing temperature (of course, there are exceptions). Depending on the substance, a difference of 10 degrees Celsius, for example, can double or quadruple the reaction speed.
How precisely refrigerated transport became possible
Knowledge of cooling with ice was available for a long time. Still, the application for specific temperature ranges required the invention of Frederick McKinley Jones, who invented and patented a mobile refrigerated unit for trucks in 1930. It's easy to gauge what a huge success that was: he co-founded Thermoking.
The pharmaceutical industry's standard temperature range is 2 – 8°C, but new technologies require new temperature levels:
Temperature ranges
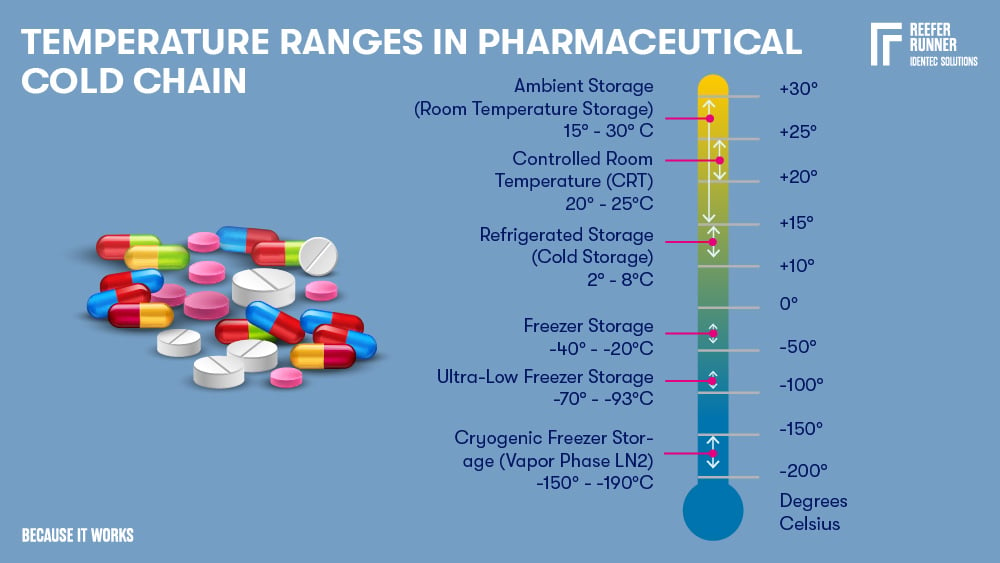
| Name | Temperature Range | Description Pharmaceuticals |
| Ambient Storage | 15-30°C | Most pharmaceuticals, including biological materials containing a preservative (e.g. alcohol or formalin) |
| Controlled Room Temperature | 20-25°C | Most pharmaceuticals, including biological materials containing a preservative (e.g. alcohol or formalin) |
| Refrigerated Storage | 2-8°C | Insulin, antidiabetics, a variety of vaccines (e.g. Flu, FSME, Typhus), eye and antibiotic ear drops, probiotics, hormonal contraceptives (e.g. ring), antibiotic suspensions, several drugs against cancer and biologicals such as proteins, coagulation factors, enzymes, antibodies, and receptors. A short-term storage option, particularly for enzymes, antibodies, and other biological reagents. |
| Freezer Storage | -40 - -20°C | Vaccines (e.g. all varicella-containing serum), biologicals including DNA and RNA. Suitable for both short-term and long-term situations. |
| Ultra-Low Freezer Storage | -93 - -70°C | Various types of molecules (e.g. proteins and nucleic acids). Vaccines (e.g. for MMR - measles-mumps-rubella). Biological samples (blood, plasma, and tissues). |
| Cryogenic Freezer Storage | -190 - -150°C | Optimal for long-term storage when all biological activity must be prevented or if a mix with preventative products is impossible. Suitable for blood, tissues, reproductive specimens, and clinical trial material. |
Understand: Storage instructions on the label decoded
Some labelling instructions are not given in an entirely exact form. WHO's Good Storage Practices (GDP) recommends the following interpretation (source):
On the label, it means:
Do not store over 30 °C from +2 °C to +30 °C
Do not store over 25 °C from +2 °C to +25 °C
Do not store over 15 °C from +2 °C to +15 °C
Do not store over 8°C from +2 °C to +8°C
Do not store below 8 °C from +8 °C to +25 °C
That's quite a lot of ranges. But how do you know which product lasts for how long and what temperature it needs?
How is the shelf life of drugs determined?
In a shelf life test (also called stability test) of drugs, their shelf life is determined experimentally by checking whether the microbiological, physical, and chemical properties comply with the specifications over the storage period. For instance, the medicinal substances' content must remain over 90% of the original content, and decomposition products are only allowed within strictly specified limits.
Furthermore, other parameters (when applicable) are checked, such as the release of the drug substance, pH value (solutions), water content (solid dosage forms), or discolouration.
The shelf life is also called the period of stability. Even if the specifications are rigorous and must be adhered to precisely, there is often a tiny buffer - the stability budget.
What is the stability budget?
During transportation, products pass through various points: manufacture, packaging, storage, and shipping. Therefore, no matter how careful you are, there are always brief phases in which the permitted temperatures are not (or cannot be) maintained.
A brief temperature spike usually does not affect a product's service life. However, pharmaceutical products are often disposed of if a standard temperature logger for temperature monitoring shows that a specific temperature limit has been exceeded.
In many cases, individual short temperature peaks do not have a negative impact on quality. However, according to the specification, the product would have to be disposed of.
In practice, the pharmaceutical industry and regulators agree that temperature fluctuations are occasionally acceptable, provided they are not too severe and do not last too long.
But what do "not too strong" and "not too long" mean? Data from temperature studies and stability testing are combined to find out. To be on the safe side, a generous margin of error is added. The result is an absolute maximum temperature and the period of time in which the storage conditions may deviate without jeopardising quality, safety, or effectiveness.
Until not too long ago, manufacturers and transporters were aware of the potential but lacked the tools to apply and monitor it. The challenge: the budget has to be divided between each stage of the transport. Only with new technologies in temperature monitoring and coordinated QA management can safe transport be guaranteed despite using the budget.
In addition to the general prevention of waste, the budget helps to avoid bottlenecks, especially when delivery conditions are not perfect and essential medicines reach the patients on time.
Read also: Container damage claim
What happens if temperature specifications are not met?
If medicines are stored at the wrong temperature, their chemical stability can be compromised. This means that, under certain circumstances, active ingredients can be broken down, or impurities can form.
Some changes can decrease effectiveness, but others can be downright dangerous. For example, if the physical properties, such as colour, texture, smell or taste, change, it usually becomes apparent that something cannot be right.
What makes things more complicated, however, is that many of these changes cannot be determined visually, olfactorily or in terms of taste, which means that a thorough investigation is required to determine whether the product can still be used.
Now, what if a product looks, smells and tastes as it should? How can you be sure that the temperature has been maintained and the stability budget has not been stretched excessively?
In a word: monitoring. Or better in two words: seamless monitoring (see: reefer alarm).
Seamless temperature monitoring
With complete temperature tracking, it can be proven that the cold chain was maintained during the entire transport. Or whether there were deviations. As long as these are within the allowed stability budget, the goods can still be used.
However, if there are significant non-conformances in the duration and level of the temperature deviation, tracking shows immediately where they happened and who is responsible for them. This saves lengthy investigations.
The monitoring holds great advantages, especially for forwarding companies and container terminals. Constant monitoring of the reefers in their area enables rapid action should the temperatures come dangerously close to the limit values.
If the power supply is also monitored in addition to the temperature, action can be taken immediately, ideally before fluctuations even occur.
The seamless monitoring applies to the entire cold chain without exception. The stability budget can only be used if you have all the information about the whole supply chain in the first place. Let's have a closer look at the pharmaceutical's journey from the manufacturer to the patient:
Essential steps along the cold chain of pharmaceuticals
Production
The cold chain sometimes begins before or during production, when components must be delivered or processed in a cooled state. Of course, the temperature of the production premises needs to be checked, monitored, and recorded.
Packaging
The packaging of medicinal products consists of three layers: primary, secondary, and tertiary.
The primary layer protects from the transmission of moisture, solvents, reactive gases, light, and microbial intrusion. This includes vials, syringes, ampoules, stoppers, closures, bottles, pouches, sachets, strips, and blisters.
The secondary layer displays the brand and product name, holds individual units or sets, and helps with logistics since it's either retail-ready, shelf-ready, or packaged as countertop display units in pouches, boxes, and trays.
The tertiary layer is for protection, handling, and transportation. It holds several secondary packages as one transport unit.
For temperature-sensitive products, there is a fourth or even several additional layers. These are mainly insulated box liners and envelopes, which can be perfectly adapted to the lower or any outer packaging size. But there are also insulated pallet covers, ice pads, and several more. Sometimes, an isolated standard container will do, but mainly reefers are used.
Dry ice usage is also possible but requires special care. In addition to complying with safety precautions for the personnel's health, dry ice must never be in contact with the product itself. Direct contact could result in freezing, significantly diminishing quality or rendering the product useless, if not dangerous.
Besides possible physical damage and incorrect temperature, incorrect storage can cause chemical changes. Thus, information such as the correct setting direction must be clearly stated on the packaging and passed on to the forwarding company. Willful manipulation is another challenge: tamper-evident packaging is a mandatory requirement in the pharmaceutical industry.
Handling
Bays for receiving and dispatch should be designed to protect production components and final products from the weather.
Storage
As with all expiration-dated products, warehouses must follow the "first expired/first out" (FEFO) principle. This is especially important for shorter shelf lives. If not otherwise specified, temperature records must be made and kept for at least one year after the product's shelf life expiry. The best practice is to position monitoring devices where fluctuations will likely happen.
Shipping
Now it gets tricky: Shipping does not mean uniform transport in one single vehicle from warehouse to warehouse. Instead, a wide variety of vehicles are used here: trucks, sometimes trains, and then, of course, ships or planes (see fact box).
What can such a journey look like?
A reefer truck takes the goods from the storage to the train station, reloading them into a refrigerated container (reefer). This reefer is loaded onto the train and travels to the container terminal.
Arriving at the container terminal, the reefer is temporarily stored on a reefer rack in the yard if it cannot be loaded onto the ship immediately.
Next step, it's loaded onto the vessel and sails away towards the port of destination, where the previous process is repeated in reverse order (see also: The role of the cold chain for Saudi import economy).
Reefer & shipping know-how
Since the reefer is such a key element in the journey, what are some of the most interesting things to know about it? There are two fundamental rules for transport in a reefer: pre-chill the cargo and do not pre-chill the reefer. Why this? Reefers are not used to cool down the cargo but to maintain its temperature. Therefore the desired temperature must already be reached before charging. And if you open a pre-cooled reefer, the incoming warm air causes condensation on the internal surfaces, potentially damaging the cargo. You don't want to produce waste or use too much electricity, so it's essential to fill the space in the reefer optimally. Of course, the max load line is there for a reason, and overfilling would block the airflow. For the same reason, one has to follow a proper loading pattern, and boxes should have air holes. If there's not enough cargo to fill the T-floor right to its edge, fillers like dunnage or cardboard are recommended. |
Storage in retail
The guidelines correspond to the ones for the production or wholesale warehouse.
Storage at point of use
At the place of use, the products must continue to be cooled just as carefully.
However, some medicines have to be thawed before use. The prescribed times must be observed here in order to guarantee the most effective use possible.
Particular attention should be paid to opened packs; some medicines, which can be stored unrefrigerated, must be refrigerated once opened (extended reading: Optimizing efficiency and safety of reefer operations).
Shipping: Air vs shipMany production facilities are concentrated in just a few locations worldwide, and the drugs must be transported over long distances to the end customer. There are two ways to do this: by plane or by ship. Numerous pharmaceutical companies have been shifting their transport to waterways for several years. The main reasons are lower costs, more efficient temperature control, and a less harmful environmental impact. The International Air Transport Organisation (IATA) stated that the share of air freight fell significantly between 2000 and 2013, from 17% to 11%. In 2018, only 0.5 million tons of pharmaceuticals were transported by plane compared to 3.5 million tons by ship. (source) AstraZeneca, for example, delivered 90% by plane in 2010; five years later, it was only 45%. The company continued to face delays as nearly 80% of shipments by air were subject to temperature fluctuations necessitating an investigation. Luckily, that only sometimes meant losing the cargo, but it did involve costs and delays. In comparison, only 10% of the shiploads experienced problems, and the losses tended to be zero. (source ; source) Of course, time-sensitive goods will continue to be delivered by the fastest means of transport, i.e. by plane. Speed is critical, especially in the fight against new diseases such as Covid-19. Another effect of Covid-19 was bottlenecks and the associated price increases for ship deliveries. Aviation benefited from this for a foreseeable period. Still, the trend turned around again in the second quarter of 2022, and companies continue to take advantage of the cheaper sea freight rates when schedules allow. |
Megatrends with impacts on the cold chain of pharmaceuticals
Sensitive biopharmaceuticals are on the rise
Biopharmaceuticals, also called biological medical products or simply known as biologics, are drugs that are manufactured in, extracted from, or semi-synthesised from biological sources. These include vaccines, blood components, allergenic substances, and tissues. In addition to their temperature sensitivity, one must consider other ambient factors, such as shock and vibration, humidity, and light during storage and shipping.
Supply of developing and emerging countries
With Covid-19, it became clear to everyone: when it comes to new, highly contagious but not highly fatal diseases, world health is only as strong as its weakest link. So it must be important to all of us to ensure the equitable distribution of medicines and vaccines to all parts of the world.
The higher societies develop, the greater the demand for better medicine. This is particularly evident in the so-called common diseases such as diabetes, which is increasing rapidly in poorer countries.
See also: Supply Chain Africa and the fight against Malaria
Stricter regulations
There are exact regulations for the transport of pharmaceuticals, such as the European Union's Good Distribution Practice (GDP) and China's Good Supply Practice of Pharmaceutical Products. In the USA, the FDA (Food and Drug Administration) has written down its provisions in several paragraphs of the Code of Federal Regulations (CFR).
They all have in common the ever-increasing need for complete documentation of compliance with the cold chain.
Increase in temperature ranges beyond 2-8°C
For some time now, there has been an increased need for transport at controlled room temperature (20-25°C) but also in ultra-low freezer storage conditions (-80 - -60°C).
Quantity AND Quality
On the one hand, the progression of widespread diseases such as diabetes means that more and more temperature-sensitive products such as insulin are being shipped.
On the other hand, small quantities of personalised medicine, such as gene therapy, call for new cold chain solutions.
New technologies
Of course, automation is at the top of the list in all areas, but there is also a lot going on in packaging, robot technology and cooling technology.
In addition to increases in efficiency and new hygiene requirements, the big issue is sustainability. The latter includes topics such as energy savings, waste prevention and less toxic or non-toxic materials.
Sustainability
A large part of the pharmaceutical industry wants to achieve carbon neutrality by 2030 with various approaches:
- Reusable packaging
- Avoidance of oversized packaging
- Avoidance of toxic packaging material
- Shift from aeroplane to ship transport
- Demand for perfectly adapted cooling solutions
The actors in the supply chain are pursuing the same goal with their means:
- Energy saving
- Switching to green energy
- Switching to electric and hydrogen vehicles
- Optimised processes
- Improved mutual coordination
- Automation and IIoT
- Environmentally friendly construction and expansion
Want to learn more about reefer monitoring?
Here is a list of major pharmaceutical products that require temperature monitoring to give an idea of the magnitude of demand and sales.
What pharmaceuticals need controlled temperature?
Insulin
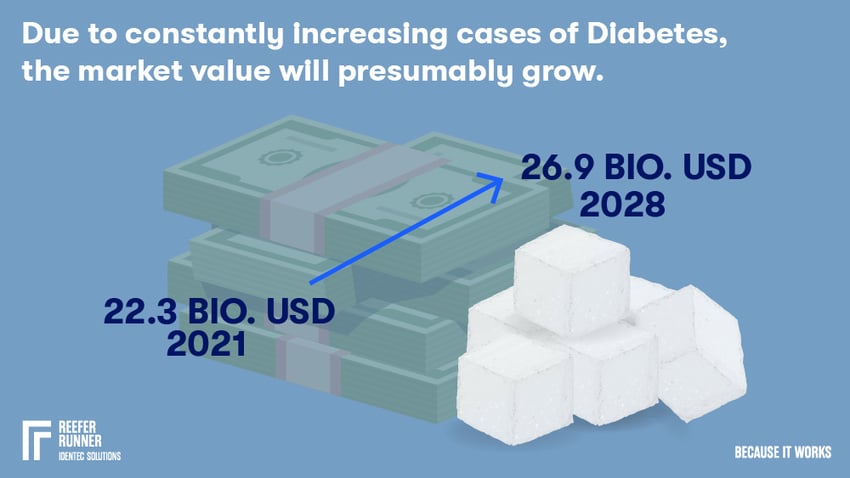
Today, about 422 Mio people worldwide have diabetes, causing 1.5 Mio deaths per year and being the primary cause of blindness, lower limb amputation, heart attacks, strokes and kidney failures. (source)
Since the discovery of insulin a hundred years ago, there has been steady improvement, and today, a large number of people are living reasonably well with the disease. But still, not everyone who needs it gets it. In Africa and Asia, in particular, an increased shortage can be observed, which is why it is crucial to ensure that as little as possible spoils during delivery in order not to make it unnecessarily expensive. Due to constantly increasing cases of this illness, the market value will presumably increase from 22.3 B USD in 2021 to 26.9 B USD in 2028. (source)
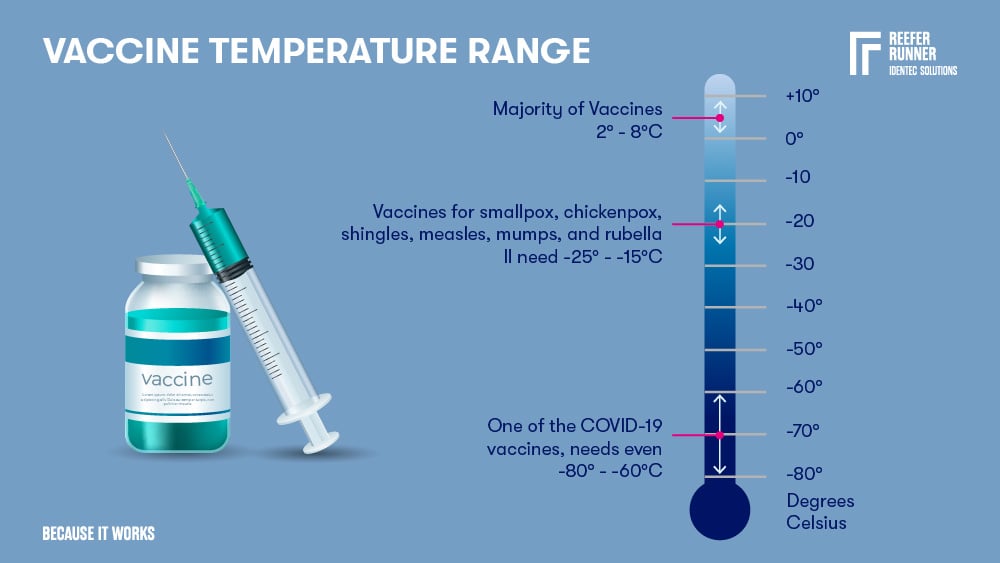
Vaccines
The majority of vaccines need a 2 – 8°C cold chain. Some vaccines for smallpox, chickenpox, shingles, measles, mumps, and rubella II need -25 - -15°C, and one that we all heard a lot of in the last two years, one of the COVID-19 vaccines needs even -80 - -60°C.
WHO's Expanded Programme on Immunization (EPI) was brought to life in 1974 to enable the immunisation of all children worldwide. The EPI is searching for solutions that don't need a cold chain, especially suitable for undeveloped countries, but it will take many years to find such replacements for all vaccines. (source)
The vaccine market grew from 5.8 billion doses valued at 38 B USD in 2019 to 16 billion doses valued at 141 B USD in 2021. (source) Of course, the main reason here is the corona pandemic. Even if this peak value will not hold, the estimate for 2028 is over 107 billion (source).
Botox
Known to most only as a wrinkle smoother, Botox has a lot more to offer. The product treats various conditions related to muscular and nerve issues. They include migraine headaches, enlarged prostate, excessive sweating, eye disorders, and more.
The global market size for Botox was 6.13 B USD in 2021 and is estimated to be close to 9 B USD in 2028. (source)
Cremes, fluids and tablets
Some creams, antibiotics in fluid form, as well as medications in tablet form, e.g. against different types of cancer, need to be refrigerated.
Asthma Sprays
The WHO estimates there are 262 Mio asthmatics worldwide. Asthma is responsible for about half a million deaths each year and is considered the most common chronic disease among children.
Ear and eye drops
Glaucoma is one of the most common reasons for blindness in people older than 60. Eye drops to treat the disease must be strictly stored at 2 - 8°C. Some antibiotic ear drops are to be stored in the same temperature range and even need to be standing upright.
Blood
Red cells and whole blood must be stored at 2 - 6°C. If the temperature sinks below 2°C, red cells become haemolysed, which means they are destroyed. Transfusing once-frozen blood can result in kidney failure and fatal bleeding.
Plasma, if it's rapidly frozen, can be stored at -40°C or colder and has a shelf life of one year.
How cold is the future of vaccines?
While solutions for more heat-resistant vaccines are being developed, on the one hand, the need for refrigerated storage and transport will most likely continue to grow. One of the main reasons for this is mRNA vaccines, which herald a new era.
The technology itself is not new, it has been known for 30 years, and clinical research has been going on for over ten years. However, the search for a remedy against Covid-19 has given further research the necessary funds. Currently, mRNA vaccines are being developed for diseases such as cancer, HIV, malaria, zika, rabies, and the flu.
These diseases are some of humanity's greatest scourges, affecting hundreds of millions of people. What a potential for improvement we finally have in our hands. However, as has already been shown in the case of Covid-19, the problem is not in the supply but in the distribution, as the WHO has publicly explained. With a production of 1.5 billion doses per month, all immunisation goals could have been met were it not for the allocation. We have to eliminate this problem as quickly as possible. The technical possibilities are there. Let's go for it.
FAQ
What is Gene Therapy?
Gene therapy is a medical procedure in which a person's genes are modified to treat or cure diseases. This involves replacing faulty genes, inactivating harmful genes, or introducing new genes.
These therapies were conceived in the late 1970s, and the first human trial took place in 1990. It treated a child with adenosine deaminase deficiency (a severe immune deficiency).
Since then, gene therapy has developed into a groundbreaking field of research, encompassing conditions such as cancer, genetic disorders (for example, spinal muscular atrophy), and rare diseases.
The products required for therapy, such as vectors, plasmid DNA, or edited cells, require strict conditions during storage and transport:
Viral vectors: Often stored frozen (-60°C to -80°C) or refrigerated (2–8°C) to ensure stability.
Plasmid DNA: Typically stable at 2–8°C for short-term storage, but freezing may be required for long-term preservation.
Edited cells: Autologous therapies (patient-specific) often require cryogenic storage (-150°C or below) in liquid nitrogen or ultra-low temperature freezers.
Enjoy our large collection of articles and whitepapers about the world of refrigerated containers!
Glossary
Hydrolysis refers to the chemical breakdown of substances by water (altering the molecular structure and properties), which can occur in temperature-sensitive products during storage or transportation. This process can lead to degradation of food quality, texture changes, and nutrient loss. In cold chain management, controlling humidity and temperature is crucial to minimise hydrolysis, particularly in products like frozen foods and pharmaceuticals. (8)
Oxidation refers to the chemical reaction that occurs when perishable goods are exposed to oxygen, leading to quality deterioration. Oxygen molecules take electrons from the food (the substrate), which creates unstable oxygen molecules, which in turn then damage the food. This process can cause rancidity in fats, colour changes, and off-flavours in foods. In temperature-sensitive products like seafood, oxidation can be accelerated by improper temperature control. (9)
Sources:
(1) https://www.expertmarketresearch.com/reports/vaccine-market
(2) https://www.paho.org/en/news/9-11-2022-who-releases-first-data-global-vaccine-market-covid-19
(3) https://en.wikipedia.org/wiki/Vaccine_storage
(4) https://www.vantagemarketresearch.com/industry-report/human-insulin-market-1911
(5) https://www.fda.gov.ph/wp-content/uploads/2021/03/World-Health-Organization-Good-Storage-Practices.pdf
(6) https://www.statista.com/statistics/263102/pharmaceutical-market-worldwide-revenue-since-2001/#:~:text=As%20of%20end-2021,%20the,what%20people%20pay%20for%20medication.
(7) https://www.fortunebusinessinsights.com/industry-reports/botulinum-toxin-market-100996
(8) H.-D. Belitz, W. Grosch, and P. Schieberle (2009): Food Chemistry. Springer.
(9) M. Shafiur Rahman (2007): Handbook of Food Preservation. CRC Press.
Note: This article was updated on the 15th of April 2025

Author
Conny Stickler, Marketing Manager Logistics
Constance Stickler holds a master's degree in political science, German language and history. She spent most of her professional career as a project and marketing manager in different industries. Her passion is usability, and she's captivated by the potential of today's digital tools. They seem to unlock endless possibilities, each one more intriguing than the last. Constance writes about automation, sustainability and safety in maritime logistics.






